In the field of injection molding production, significant differences exist between medical molds and ordinary molds in terms of design, manufacturing, and application. These disparities stem from the stringent safety, functionality, and hygiene standards required in the medical industry. This article systematically analyzes the similarities and differences between the two from five dimensions: material selection, process control, design requirements, quality inspection, and production environment.
1. Material Selection: Core Differences in Biocompatibility and Corrosion Resistance
Medical molds must meet triple standards of biocompatibility, corrosion resistance, and mechanical properties. Materials such as medical-grade stainless steel (e.g., 316L), titanium alloys, and polyetheretherketone (PEEK) are mainstream choices due to their corrosion resistance, non-toxicity, and compatibility with human tissues. For example, molds for implantable medical devices must adhere to ISO 10993 standards to ensure no toxic reactions occur during long-term contact with human tissues.
In contrast, ordinary molds prioritize cost-effectiveness and processability in material selection, such as general-purpose plastics (ABS, PP) or carbon steel and aluminum alloys. These materials do not require biocompatibility certification but must meet product functional requirements. For instance, molds for household appliance casings may prefer impact-resistant engineering plastics.
2. Process Control: Dual Challenges of Precision and Parameter Stability
Injection molding for medical molds requires strict control of temperature, pressure, speed, and holding time. Take the housing of a cardiac pacemaker as an example: the barrel temperature must be precise within ±2°C, and injection pressure fluctuations must be controlled within ±1% to avoid performance risks caused by wall thickness tolerances. Additionally, the design of the mold cooling system must optimize heat transfer efficiency to reduce dimensional deviations caused by thermal expansion and contraction.
For ordinary molds, process parameters allow a wider fluctuation range. For example, injection speed for automotive interior molds may tolerate ±5% error, and surface quality only needs to meet visual defect-free standards.
3. Design Requirements: Balancing Precision Structure and Functional Adaptation
Medical mold design must balance high precision, complex structures, and hygiene standards. For instance, molds for surgical instrument handles require pin-point gating and in-mold cutting technologies to avoid contamination risks from manual trimming. The parting surface clearance must be less than 0.01mm, and the ejection system’s fit clearance must be less than 0.01mm to ensure products are free from burrs or flash. Moreover, the mold core for transparent medical components must be polished to Ra0.2μm or higher to meet optical-grade surface requirements.
Ordinary mold design prioritizes production efficiency and cost optimization. For example, molds for daily necessities may adopt simplified parting surface designs, allowing larger gaps to reduce machining difficulty. Gate positions may be selected in non-critical areas to minimize post-processing steps.
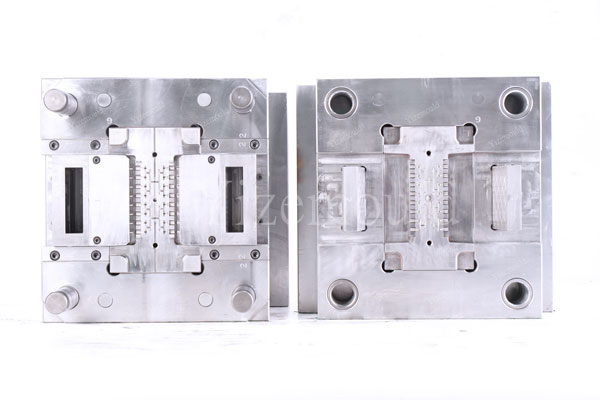
4. Quality Inspection: Full-Process Traceability and Risk Control Differences
Medical mold injection-molded products must pass multi-dimensional quality inspections, including:
-
Material composition analysis to verify compliance with ISO 13485 or FDA standards;
-
Dimensional accuracy testing using coordinate measuring machines to ensure critical dimensions are within ±0.005mm;
-
Biocompatibility testing, such as cytotoxicity tests and sensitization tests;
-
Sterility validation through ethylene oxide sterilization or gamma irradiation to ensure product sterility.
Quality inspections for ordinary molds typically only include visual checks, dimensional measurements, and basic performance tests (e.g., tensile strength), without biocompatibility or sterility verification.
5. Production Environment: Stringent Requirements for Cleanliness and Process Hygiene
Medical mold injection molding must be conducted in Class 10,000 or higher cleanrooms, with precise temperature and humidity control (e.g., 22±2°C, 50±10%RH) to prevent microbial contamination. Molds must undergo regular sterilization via high-temperature, high-pressure steam or use medical-grade release agents. Operators must wear protective clothing, gloves, and masks.
Ordinary mold injection molding workshops typically only require basic dust-proofing, with broader temperature and humidity control ranges (e.g., 15-30°C, 30-70%RH). Operators do not need special protective equipment.
Conclusion: The "High Threshold" and "High Value" of Medical Molds
The core difference between medical molds and ordinary molds lies in their extreme pursuit of safety, functionality, and hygiene standards. Medical molds ensure product compliance with stringent medical industry requirements through material upgrades, process optimization, design innovation, and full-process quality control, but this comes with higher manufacturing costs and technical barriers. In contrast, ordinary molds prioritize efficiency and cost balance, making them suitable for scenarios with lower precision and hygiene requirements. With the advancement of medical technology, the customized and intelligent trends of medical molds will further highlight their irreplaceability.














 Home
Home
