When venturing into the realm of medical plastic injection molding, the primary focus is on medical safety, encompassing detailed classification of medical devices, careful selection of materials for medical consumables, consideration of device-human contact points, biocompatibility of plastics, production environment and regulatory requirements for medical devices, as well as the production management system for medical consumables. Below is an elaboration on these key points:
1. Classification of Medical Devices
The National Medical Products Administration provides a thorough classification of medical consumables, covering aspects such as intended use, passive and active medical devices, invasive and non-invasive devices, reusable and implantable devices, devices that contact different parts of the human body, duration of use, and those involving the skin, body cavities, wounds, tissues, circulatory system, central nervous system, etc.
2. Selection of Medical Materials
Based on the classification of medical devices and specific usage environments, duration of use, and tissue contact, it is essential to carefully select plastics with corresponding biocompatibility. For Class I medical devices that do not directly contact the human body, material selection may lean towards the requirements for electronic products. Meanwhile, there are various plastics specifically designed for the medical industry, such as those used in in-vitro diagnostics, medical consumables, and medical equipment, and these choices should be based on thorough market research and professional consultation.
3. Commonly Used Plastics in the Medical Industry & Partner Selection
During the material selection process, it is crucial to refer to past successful cases and consider the aforementioned multiple factors. Professional teams like Bashan Technology can provide valuable support in this area, helping to save time and optimize costs. For medical injection molding needs, seeking professional consultation is a wise decision.
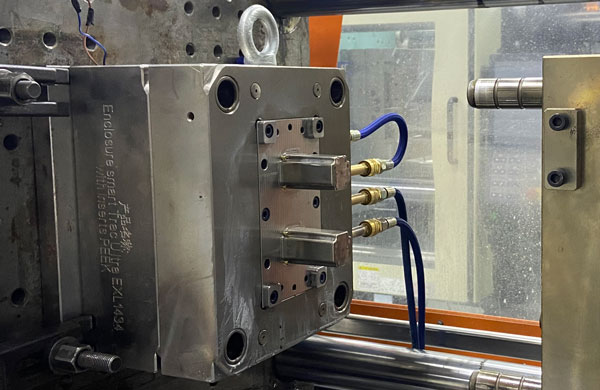
4. Production of Medical Moulds & Key Considerations
Medical moulds differ significantly from ordinary electronic products, automotive products, and consumer goods. In the development of medical moulds, special attention must be paid to reliability and stability, ensuring no oil contamination during the injection molding process, and optimizing mould structure. Furthermore, it is important to avoid increased later-stage time and costs due to initial cost-saving measures in mould development. Choosing a partner with rich experience can provide valuable support in aspects such as steel material selection and structure optimization, thereby reducing overall development costs and time.
5. Medical Injection Molding Production
The production of medical devices requires determining whether medical-grade clean injection molding is needed based on their classification and specific uses. The production process must adhere to the standards of internationally certified or GMP-certified medical system enterprises. Safety and quality control in medical injection molding encompass multiple links, from mould development and production, mould structure reliability, injection molding process supervision, to the packaging of medical injection molded parts.
















 Home
Home
